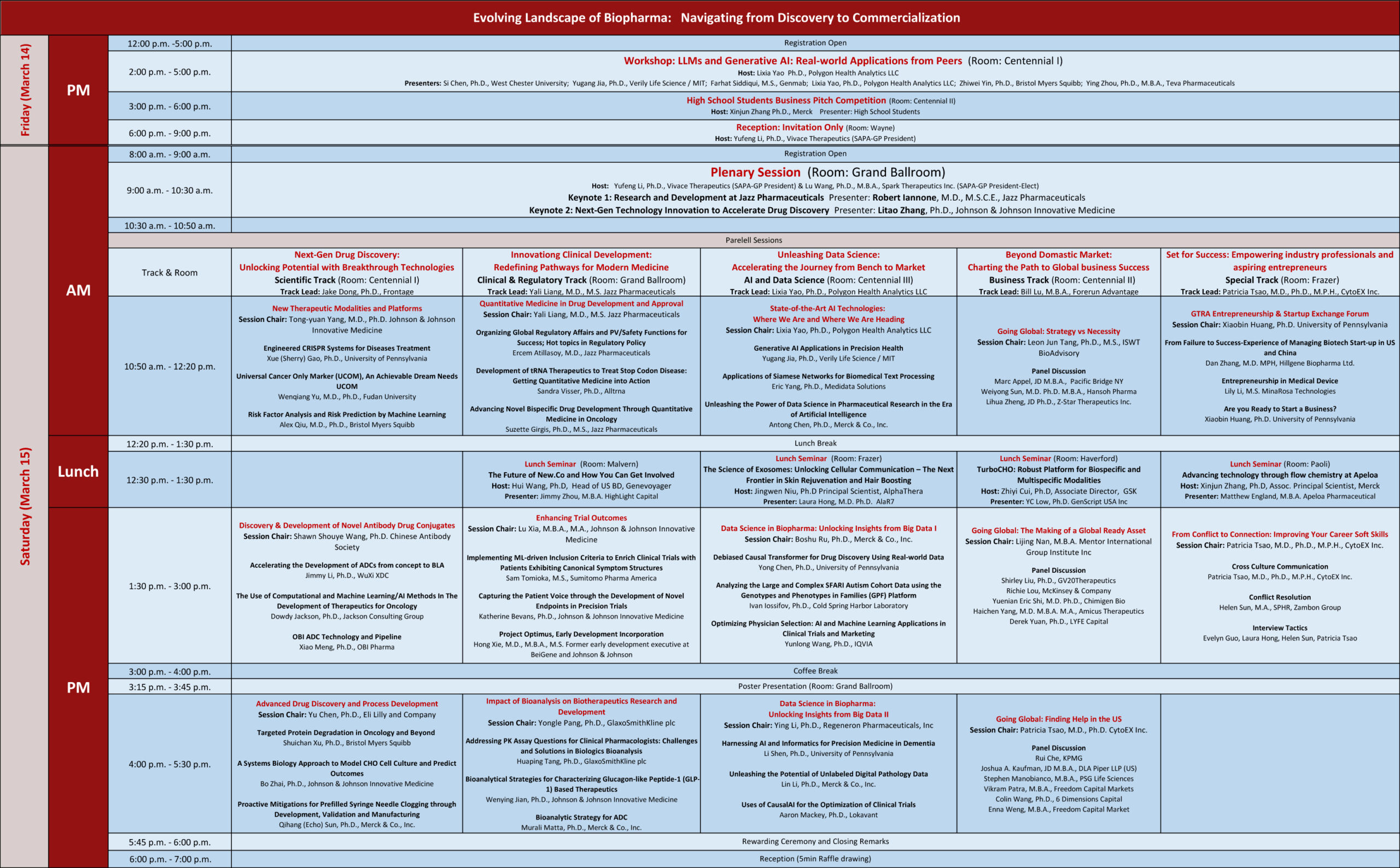
Schedule at a Glance
Friday, March 14, 2025
| 12:00-5:00 | Registration Open |
| 2:00 – 5:00 | Workshop: LLMs and Generative AI: Real-world Applications from Peers Host: Lixia Yao Room: Centennial I Presenter: Yugang Jia, Zhiwei Yin, Si Chen, Ying Zhou, Lixia Yao, Farhat Siddiqui |
| 3:00 – 6:00 | High School Students Business Pitch Competition: Host: Xinjun Zhang Room: Centennial II Presenter: High School Students |
| 6:00 – 9:00 | President Reception: Invitation Only Host: Yufeng Li |
Saturday, March 15, 2025
| 8:00 – 9:00 | Registration Open |
| 9:00 – 10:30 |
Plenary SessionHost: Yufeng Li & Lu Wang |
| 10:30 – 10:50 | Coffee Break |
| 10:50 – 12:20 |
Parallel Sessions 1 |
| 12:20 – 1:30 | Lunch Break |
| 12:30 – 1:30 | Lunch Seminar TurboCHO: Robust Platform for Biospecific and Multispecific Modalities YC Low, Ph.D. GenScript (Room: Haverford, Host: Zhiyi Cui) The Future of New.Co and How You Can Get Involved Jimmy Zhou, M.B.A. HighLight Capital (Room: Malvern, Host: Hui Wang) TurboCHO: Robust Platform for Biospecific and Multispecific Modalities Laura Hong, M.D. Ph.D. AlaR7 (Room: Frazer, Host: Jingwen Niu) Advancing technology through flow chemistry at Apeloa Matthew England Apeloa Pharmaceutical (Room: Haverford, Host: Xinjun Zhang) |
| 1:30 – 3:00 |
Parallel Sessions 2 |
| 3:00 – 4:00 | Coffee Break |
| 3:15 – 3:45 | Poster Presentation |
| 4:00 – 5:30 |
Parallel Sessions 3 |
| 5:30-5:45 | Rewarding Ceremony and Closing Remarks |
| 5:45-6:45 | Reception (5min Raffle drawing) |
Parallel Sessions:
Parallel Session 1 |
Quantitative Medicine in Drug Development and Approval |
| 10:50-11:20 | Organizing Global Regulatory Affairs and PV/Safety Functions for Success; Hot topics in Regulatory Policy Ercem Atillasoy, M.D., Jazz Pharmaceuticals |
| 11:20-11:50 | Development of tRNA therapeutics to Treat Stop Codon Disease: Getting Quantitative Medicine into Action Sandra Visser, Ph.D., Alltrna |
| 11:50-12:20 | Advancing Novel Bispecific Drug Development Through Quantitative Medicine in Oncology Suzette Girgis, Ph.D. M.S., Jazz Pharmaceuticals |
Parallel Session 2 |
Enhancing Trial Outcomes |
| 1:30-2:00 | Implementing ML-driven inclusion criteria to enrich clinical trials with patients exhibiting canonical symptom structures Sam Tomioka, M.S., Sumitomo Pharma America |
| 2:00-2:30 | Capturing the patient voice through the development of novel endpoints in precision trials Katherine Bevans, Ph.D., Johnson & Johnson Innovative Medicine |
| 2:30-3:00 | Project Optimus, Early Development Incorporation Hong Xie, M.D., M.B.A., M.S. Former early development executive at BeiGene and Johnson & Johnson |
Parallel Session 3 |
Impact of Bioanalysis on Biotherapeutics Research and Development |
| 4:00-4:30 | Addressing PK assay questions for clinical pharmacologists: challenges and solutions in Biologics bioanalysis Huaping Tang, Ph.D., GlaxoSmithKline plc |
| 4:30-5:00 | Bioanalytical strategies for characterizing glucagon-like peptide-1 (GLP-1) based therapeutics Wenying Jian, Ph.D., Johnson & Johnson Innovative Medicine |
| 5:00-5:30 | Bioanalytic strategy for ADC Weifeng Xu, Ph.D., Merck & Co., Inc. |
Parallel Session 1 |
New Therapeutic Modalities and Platforms |
| 10:50-11:20 | Engineered CRISPR systems for diseases treatment Xue (Sherry) Gao, Ph.D., University of Pennsylvania |
| 11:20-11:50 | Universal Cancer Only Marker (UCOM), An Achievable Dream Needs U COM Wenqiang Yu, M.D., Ph.D., Fudan University |
| 11:50-12:20 | Risk factor analysis and risk prediction by machine learning Alex Qiu, M.D., Ph.D., Bristol Myers Squibb |
Parallel Session 2 |
Discovery & Development of Novel Antibody Drug Conjugates |
| 1:30-2:00 | Accelerating the development of ADCs from concept to BLA Jimmy Li, Ph.D., WuXi XDC |
| 2:00-2:30 | The Use of Computational and Machine Learning/AI Methods In The Development of Therapeutics for Oncology Dowdy Jackson, Ph.D., Jackson Consulting Group |
| 2:30-3:00 | OBI ADC Technology and Pipeline Xiao Meng, Ph.D., OBI Pharma |
Parallel Session 3 |
Advanced Drug Discovery and Processing Development |
| 4:00-4:30 | Targeted Protein Degradation in Oncology and Beyond Shuichan Xu, Ph.D., Bristol Myers Squibb |
| 4:30-5:00 | A Systems Biology Approach to Model CHO Cell Culture and Predict Outcomes Bo Zhai, Ph.D., Johnson & Johnson Innovative Medicine |
| 5:00-5:30 | Proactive Mitigations for Prefilled Syringe Needle Clogging through Development, Validation and Manufacturing. Qihang (Echo) Sun, Ph.D., Merck & Co., Inc. |
Parallel Session 1 |
Going Global: Strategy vs Necessity |
| 10:50-12:20 | Panel Discussion Marc Appel, JD M.B.A., Pacific Bridge NY Jeff He, M.B.A M.A B.A., Genmab Weiyong Sun, M.D. Ph.D. M.B.A., Hansoh Pharma Lihua Zheng, JD Ph.D., Z-Star Therapeutics Inc. |
Parallel Session 2 |
Going Global: The Making of a Global Ready Asset |
| 1:30-3:00 | Panel Discussion Shirley Liu, Ph.D., GV20Therapeutics Richie Lou, McKinsey & Company Yuenian Eric Shi, M.D. Ph.D., Chimigen Bio Haichen Yang, M.D. M.B.A. M.A., Amicus Therapeutics Derek Yuan, Ph.D., LYFE Capital |
Parallel Session 3 |
Going Global: Finding Help in the US |
| 4:00-5:30 | Panel Discussion Joshua A. Kaufman, JD M.B.A., DLA Piper LLP (US) Stephen Manobianco, M.B.A., PSG Life Sciences Vikram Patra, M.B.A., Freedom Capital Market Colin Wang, Ph.D., 6 Dimensions Capital Enna Weng, M.B.A., Freedom Capital Market |
Parallel Session 1 |
State-of-the-Art AI Technologies: Where We Are and Where We Are Heading |
| 10:50-11:20 | Generative AI applications in precision health Yugang Jia, Ph.D., Verily Life Science / MIT |
| 11:20-11:50 | Applications of Siamese Networks for Biomedical Text Processing Eric Yang, Ph.D., Medidata Solutions |
| 11:50-12:20 | Unleashing the Power of Data Science in Pharmaceutical Research in the Era of Artificial Intelligence Antong Chen, Ph.D., Merck & Co., Inc. |
Parallel Session 2 |
Data Science in Biopharma: Unlocking Insights from Big Data I |
| 1:30-2:00 | Debiased causal transformer for drug discovery using real-world data Yong Chen, Ph.D., University of Pennsylvania |
| 2:00-2:30 | Analyzing the large and complex SFARI autism cohort data using the Genotypes and Phenotypes in Families (GPF) platform Ivan Iossifov, Ph.D., CSHL |
| 2:30-3:00 | Optimizing Physician Selection: AI and Machine Learning Applications in Clinical Trials and Marketing Yunlong Wang, Ph.D., IQVIA |
Parallel Session 3 |
Data Science in Biopharma: Unlocking Insights from Big Data II |
| 4:00-4:30 | Harnessing AI and Informatics for Precision Medicine in Dementia Li Shen, Ph.D., University of Pennsylvania |
| 4:30-5:00 | Unleashing the potential of unlabeled digital pathology data Lin Li, Ph.D., Merck & Co., Inc. |
| 5:00-5:30 | Uses of CausalAI for the Optimization of Clinical Trials Aaron Mackey, Ph.D., Lokavant |
Parallel Sessions 1 |
GTRA Entrepreneurship & Startup Exchange Forum |
| 10:50-11:20 | From Failure to Success-Experience of Managing Biotech Start-up in US and China Dan Zhang, M.D. MPH, Hillgene Biopharma Ltd. |
| 11:20-11:50 | Entrepreneurship in Medical Device Lily Li, M.S. MinaRosa Technologies |
| 11:50-12:20 | Are you ready to start a business? Xiaobin Huang, Ph.D. University of Pennsylvania |
Parallel Sessions 2 |
From conflict to connection: improving your career soft skills |
| 1:30-2:00 | Cross Culture Communication Patricia Tsao, M.D., Ph.D., M.P.H., CytoEX Inc. |
| 2:00-2:30 | Conflict Resolution Helen Sun, M.A., SPHR, Zambon Group |
| 2:30-3:00 | Interview Tactics Evelyn Guo, Helen Sun, Patricia Tsao |
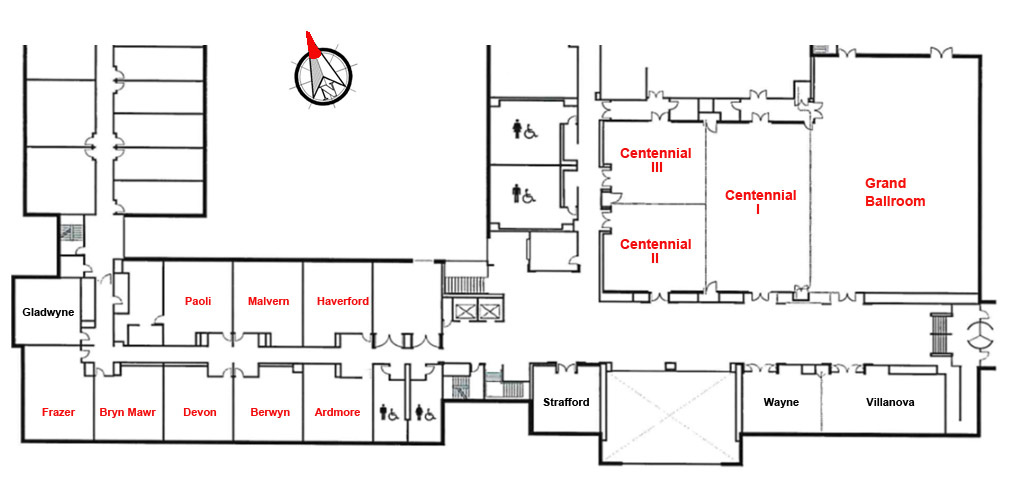
Meeting rooms map: